Organic Chemistry for Life Science
Developing new methodologies for the construction of a broad range of molecules significant in life sciences
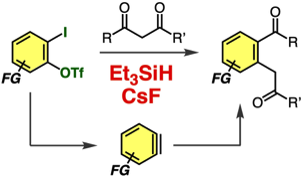
Acylalkylation of Arynes Generated from o-Iodoaryl Triflates with Hydrosilanes and Cesium Fluoride
◆
M. Minoshima, K. Uchida, Y. Nakamura, T. Hosoya,
*S. Yoshida
Org. Lett. 2021, 23, 1868.
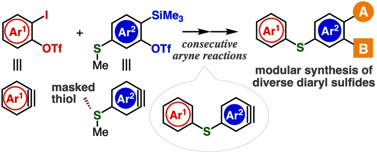
Diverse diaryl sulfide synthesis through consecutive aryne reactions
◆
H. Nakajima, Y. Hazama, Y. Sakata, K. Uchida, T. Hosoya, *S. Yoshida
Chem. Commun., 2021, 57, 2621.
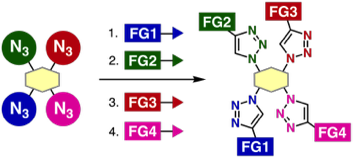
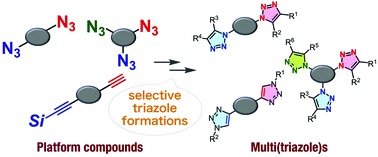
Assembly of four modules onto a tetraazide platform by consecutive 1,2,3-triazole formations
◆
*S. Yoshida, Y. Sakata, Y. Misawa, T. Morita, T. Kuribara, H. Ito, Y. Koike, I. Kii, *T. Hosoya
Chem. Commun., 2021, 57, 899.
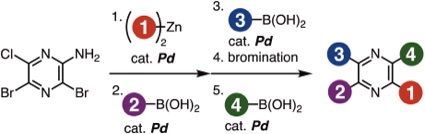
Facile Synthesis of Tetraarylpyrazines by Sequential Cross-coupling Approach
◆
Y. Nishiyama, K. Akiyama, Y. Sakata, T. Hosoya, *S. Yoshida
Chem. Lett. 2021, 50, 180.
https://doi.org/10.1246/cl.200715
Selected as Editor’s choice; Open Access
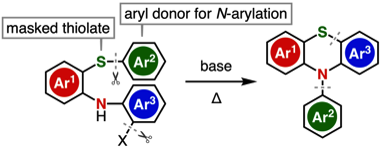
Transition-Metal-Free Synthesis of N-Arylphenothiazines through an N- and S-Arylation Sequence
◆
T. Matsuzawa, T. Hosoya, *S. Yoshida
Org. Lett. 2021, 23, 2347.
◆
Sequential conjugation methods based on triazole formation and related reactions using azides
*S. Yoshida
Recent review article
Recent original papers
Faculty of Advanced Engineering
Department of Biological Science and Technology
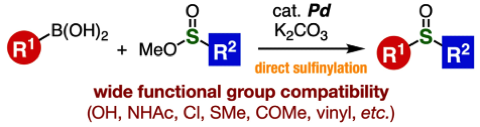
Palladium-Catalyzed Sulfinylation of Aryl- and Alkenylborons with Sulfinate Esters
◆
M. Suzuki, K. Kanemoto, Y. Nakamura, T. Hosoya,
S. Yoshida*
Org. Lett. 2021, in press.