Publication
Original Paper (2020~present / 2016~2019 / before 2015)/Review, book, etc
Faculty of Advanced Engineering
Department of Biological Science and Technology
Acylalkylation of Arynes Generated from o-Iodoaryl Triflates with Hydrosilanes and Cesium Fluoride
◆ 109
M. Minoshima, K. Uchida, Y. Nakamura, T. Hosoya,
*S. Yoshida
Org. Lett. 2021, 23, 1868.
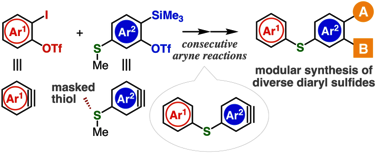
Diverse diaryl sulfide synthesis through consecutive aryne reactions
◆ 107
H. Nakajima, Y. Hazama, Y. Sakata, K. Uchida, T. Hosoya, *S. Yoshida
Chem. Commun. 2021, 57, 2621.
S1PR3–G12-biased agonist ALESIA targets cancer metabolism and promotes glucose starvation
◆ 108
M. Toyomoto, A. Inoue, K. Iida, M. Denawa, I. Kii, F. Marie, N. Kadji, T. Kishi, D. Im, T. Shimamura, H. Onogi, S. Yoshida, S. Iwata, J. Aoki, T. Hosoya, *M. Hagiwara
Cell Chem. Biol. 2021, in press.
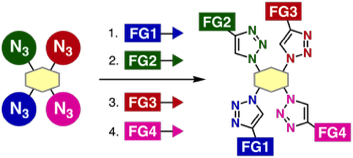
Assembly of four modules onto a tetraazide platform by consecutive 1,2,3-triazole formations
◆ 106
*S. Yoshida, Y. Sakata, Y. Misawa, T. Morita, T. Kuribara, H. Ito, Y. Koike, I. Kii, *T. Hosoya
Chem. Commun. 2021, 57, 899.
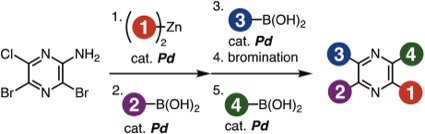
Facile Synthesis of Tetraarylpyrazines by Sequential Cross-coupling Approach
◆ 105
Y. Nishiyama, K. Akiyama, Y. Sakata, T. Hosoya, *S. Yoshida
Chem. Lett. 2021, 50, 180.
https://doi.org/10.1246/cl.200715
Selected as Editor’s choice; Open Access
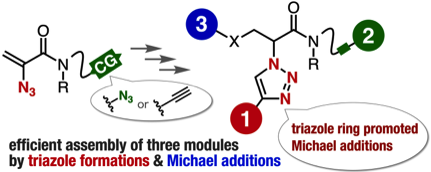
2-Azidoacrylamides as compact platforms for efficient modular synthesis
◆ 104
H. Takemura, S. Goto, T. Hosoya, *S. Yoshida
Chem. Commun. 2020, 56, 15541.
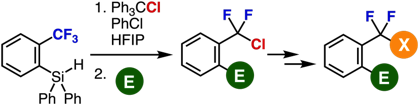
Single C−F Transformations of o‑Hydrosilyl Benzotrifluorides with Trityl Compounds as All-in-One Reagents
◆ 103
R. Idogawa, Y. Kim, K. Shimomori, T. Hosoya,
*S. Yoshida
Org. Lett. 2020, 22, 9292.
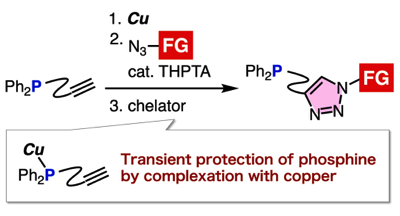
Triazole formation of phosphinyl alkynes with azides through transient protection of phosphine by copper
◆ 102
N. Terashima, Y. Sakata, T. Meguro, T. Hosoya,
*S. Yoshida
Chem. Commun. 2020, 56, 14003.
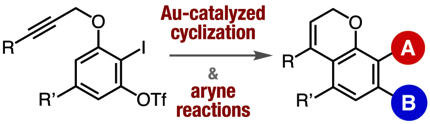
Synthesis of Functionalized Benzopyran/Coumarin-Derived Aryne Precursors and Their Applications
◆ 101
Y. Nakamura, Y. Sakata, T. Hosoya, *S. Yoshida
Org. Lett. 2020, 22, 8505.
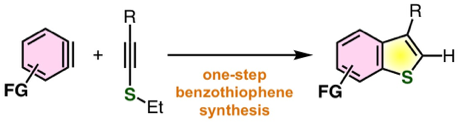
One-step synthesis of benzo[b]thiophenes by aryne reaction with alkynyl sulfides
◆ 100
T. Matsuzawa, T. Hosoya, *S. Yoshida
Chem. Sci. 2020, 11, 9691.
https://doi.org/10.1039/D0SC04450D
Open Access; 化学工業日報; Highlighted in Chemistry Views
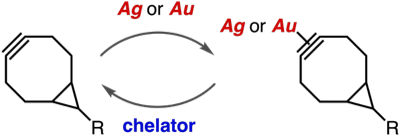
(Hexafluoroacetylacetonato)copper(I)–cycloalkyne complexes as protected cycloalkynes
◆ 99
N. Makio, T. Kuribara, K. Adachi, Y. Hatakeyama,
T. Meguro, Y. Sakata, K. Igawa, K. Tomooka, T. Hosoya,
*S. Yoshida
Selective strain-promoted azide–alkyne cycloadditions through transient protection of bicyclo[6.1.0]nonynes with silver or gold
◆ 98
K. Adachi, T. Meguro, Y. Sakata, K. Igawa, K. Tomooka,
T. Hosoya, *S. Yoshida
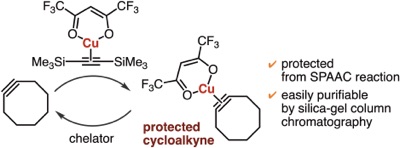
Synthesis of multisubstituted cycloalkenes through carbomagnesiation of strained cycloalkynes
◆ 97
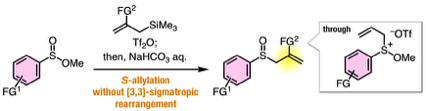
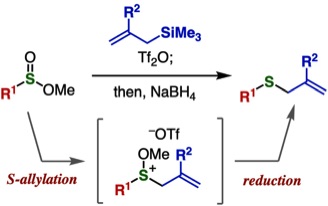
Y. Tamura, Y. Minami, Y. Nishiyama, Y. Sakata, F. Karaki,
T. Hosoya, *S. Yoshida
One-pot Synthesis of Allyl Sulfides from Sulfinate Esters and Allylsilanes through Reduction of Alkoxysulfonium Intermediates
◆ 96
A. Kobayashi, T. Matsuzawa, T. Hosoya, *S. Yoshida
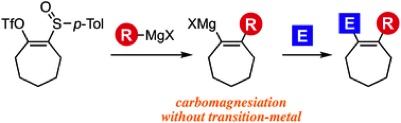
Aryne Reaction and Cross-Coupling Approach for the Synthesis of Diverse N-Arylphenylalanine Derivatives
◆ 95
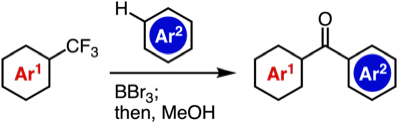
T. Kobayashi, T. Hosoya, *S. Yoshida
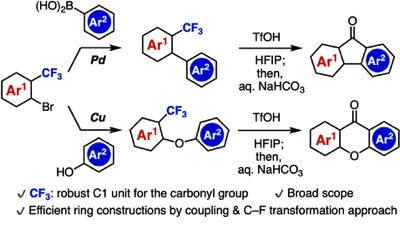
Synthesis of Diverse Aromatic Ketones through C–F Cleavage of Trifluoromethyl Group
◆ 94
M. Ikeda, T. Matsuzawa, T. Morita, T. Hosoya,
*S. Yoshida
Chem. Eur. J. 2020, 26, 12333.
Synthesis of benzyl sulfides via substitution reaction at the sulfur of phosphinic acid thioesters
◆ 93
Y. Nishiyama, T. Hosoya, *S. Yoshida
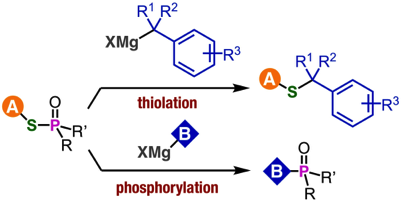
Sulfoxide Synthesis from Sulfinate Esters under Pummerer-like Conditions
◆ 92
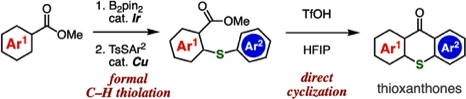
A. Kobayashi, T. Matsuzawa, T. Hosoya, *S. Yoshida
Synthesis of Thioxanthones through Formal C–H Thiolation of Benzoic Acid Esters and Acid-mediated Direct Cyclization
◆ 91
K. Mutsuura, Y. Sakata, K. Uchida, T. Hosoya,
*S. Yoshida
A novel yellow fluorescent protein of recombinant apoPholasin with dehydrocoelenterazine
◆ 89
S. Inouye, Y. Miura-Sahara, R. Iimori, Y. Sakata,
Y. Hazama, S. Yoshida, M. Nakamura, T. Hosoya
Biochem. Biophys. Res. Commun. 2020, 526, 404.
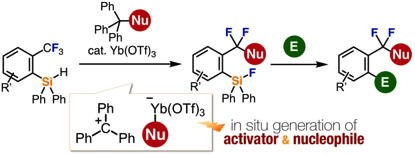
Functionalization of a Single C–F Bond of Trifluoromethylarenes Assisted by an ortho-Silyl Group Using a Trityl-Based All-in-One Reagent with Ytterbium Triflate Catalyst
◆ 88
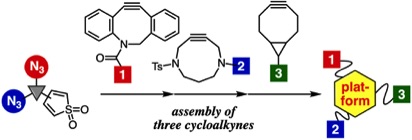
Facile assembly of three cycloalkyne-modules onto a platform compound bearing thiophene S,S-dioxide moiety and two azido groups
◆ 90
T. Meguro, Y. Sakata, T. Morita, T. Hosoya, *S. Yoshida
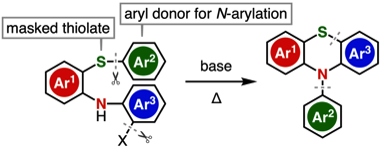
Transition-Metal-Free Synthesis of N-Arylphenothiazines through an N- and S-Arylation Sequence
◆ 110
T. Matsuzawa, T. Hosoya, *S. Yoshida
Org. Lett. 2021, 23, 2347.
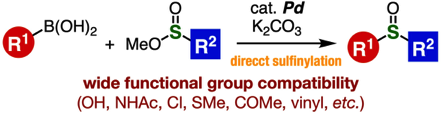
Palladium-Catalyzed Sulfinylation of Aryl- and Alkenylborons with Sulfinate Esters
◆ 111
M. Suzuki, K. Kanemoto, Y. Nakamura, T. Hosoya,
*S. Yoshida
Org. Lett. 2021, 23, 3793..
◆ 112
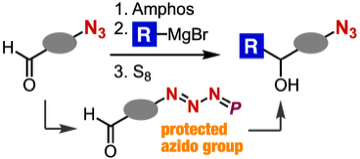
Nucleophilic transformations of azido-containing carbonyl compounds via protection of azido group
T. Aimi, T. Meguro, A. Kobayashi, *T. Hosoya, *S. Yoshida
Chem. Commun. 2021, 57, 6062.
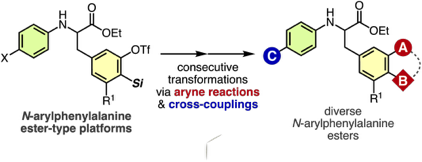
◆ 113
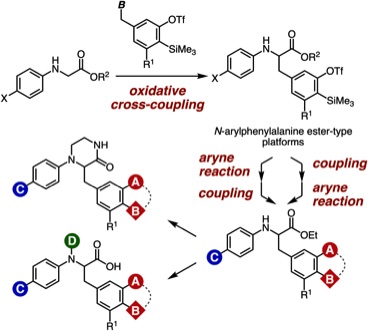
Facile Synthetic Methods for Diverse N-Arylphenylalanine Derivatives via Transformations of Aryne Intermediates and Cross-Coupling Reactions
T. Kobayashi, T. Hosoya, *S. Yoshida
Bull. Chem. Soc. Jpn. 2021, 94, 1823.
◆ 114
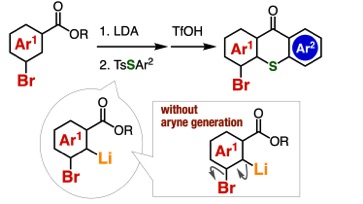
Thioxanthone Synthesis from Benzoic Acid Esters through Directed ortho-Lithiation
A. Kobayashi, T. Matsuzawa, T. Hosoya, *S. Yoshida
Chem. Lett. 2021, 50, 1624. [Selected as Editor's Choice (優秀論文)]
◆ 115
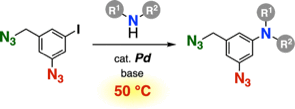
Synthesis of Azidoanilines by the Buchwald–Hartwig Amination
Y. Sakata, S. Yoshida, *T. Hosoya
J. Org. Chem. 2021, 86, 15674.
◆ 116
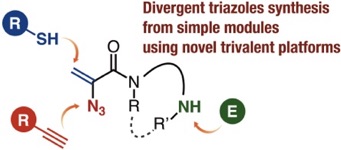
Multicomponent click assembly through 2-azidoacrylamides
having a nucleophilic amino group
H. Takemura, G. Orimoto, A. Kobayashi, T. Hosoya,
S. Yoshida*
◆ 117
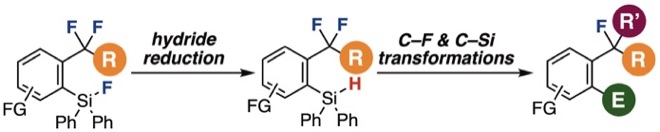
Hydride reduction of o-(fluorosilyl)benzodifluorides for subsequent C–F transformations
R. Idogawa, A. Kobayashi, Y. Kim, K. Shimomori, T. Hosoya, S. Yoshida*
Chem. Commun. 2022, 58, 3521.
◆ 118
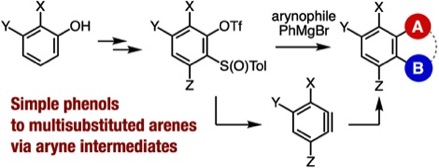
Synthesis of Multisubstituted Benzenes from Phenols via Multisubstituted Benzynes
A. Nagai, A. Kobayashi, Y. Sakata, Y. Minami,
K. Uchida, T. Hosoya, S. Yoshida*
Synthesis 2022, 54, 5017.
[Special topic: Aryne Chemistry in Synthesis]
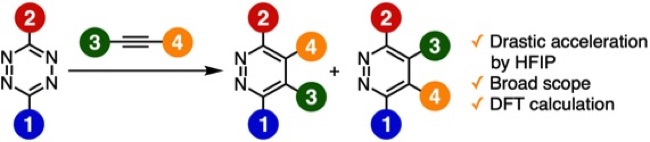
◆ 120
Pyridazine Synthesis from 1,2,4,5-Tetrazines and Alkynes in 1,1,1,3,3,3-Hexafluoro-2-propanol through the Inverse Electron Demand Diels–Alder Reaction
C. Yamamoto, M. Suzuki, S. Yoshida*
Bull. Chem. Soc. Jpn. 2022, 95, 1741. [BCSJ award article]
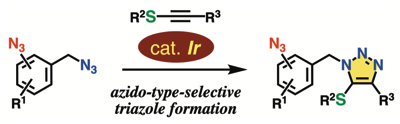
◆ 119
Azido-type-selective triazole formation by iridium-catalyzed cycloaddition with thioalkynes
K. Sugiyama, Y. Sakata, T. Niwa, S. Yoshida, *T. Hosoya
Chem. Commun. 2022, 58, 6235.
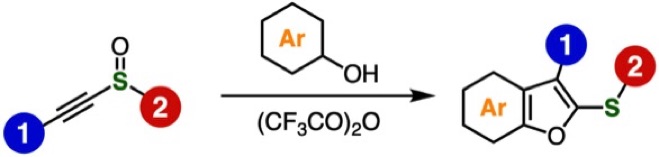
◆ 121
Synthesis of benzo[b]furans from alkynyl sulfoxides and phenols by the interrupted Pummerer reaction
A. Kobayashi, T. Matsuzawa, T. Hosoya, S. Yoshida*
RSC Adv. 2023, 13, 839.
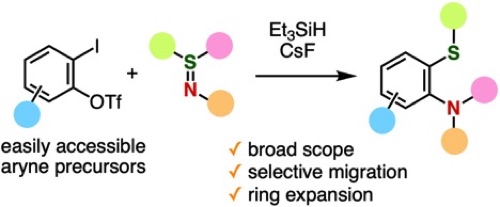
◆ 122
Migrative Thioamination of Aryne Intermediates Generated from o-Iodoaryl Triflates
S. Tabata, M. Minoshima, A. Kobayashi, T. Hosoya, S. Yoshida*
◆ 123
Fluorenones and Xanthones through Intramolecular C–F Arylation.
S. Hamada, S. Yoshida*
Bull. Chem. Soc. Jpn. 2023, in press.