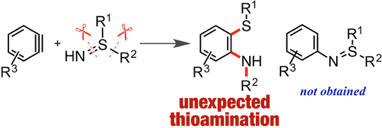
Direct Thioamination of Arynes via Reaction with Sulfilimines and Migratory N-Arylation
◆ 43
S. Yoshida, T. Yano, Y. Misawa, Y. Sugimura, K. Igawa,
S. Shimizu, K. Tomooka, *T. Hosoya
J. Am. Chem. Soc. 2015, 137, 14071.
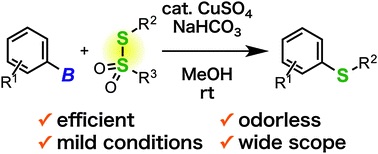
A mild and facile synthesis of aryl and alkenyl sulfides via copper-catalyzed deborylthiolation of organoborons with thiosulfonates
◆ 42
*S. Yoshida, Y. Sugimura, Y. Hazama, Y. Nishiyama,
T. Yano, S. Shimizu, *T. Hosoya
Chem. Commun. 2015, 51, 16613.
https://doi.org/10.1039/C5CC07463K
Selected as inside back cover; Highlighted in Chemistry View
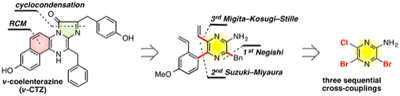
Concise Synthesis of v-Coelenterazines
◆ 41
*T. Hosoya, R. Iimori, S. Yoshida, Y. Sumida,
Y. Sahara-Miura, J.-i. Sato, S. Inouye
Org. Lett. 2015, 17, 3888.
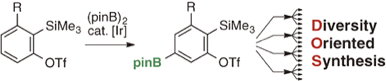
Facile Synthesis of Diverse Multisubstituted ortho-Silylaryl Triflates via C–H Borylation
◆ 40
S. Yoshida, K. Shimomori, T. Nonaka, *T. Hosoya
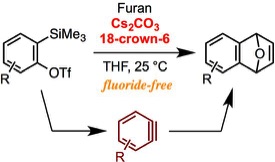
An Alternative Method for Generating Arynes from ortho-Silylaryl Triflates: Activation by Cesium Carbonate in the Presence of a Crown Ether
◆ 39
S. Yoshida, Y. Hazama, Y. Sumida, T. Yano,
*T. Hosoya
Molecules 2015, 20, 10131.
https://doi.org/10.3390/molecules200610131
Special Issue: Development and Application of Aryne Chemistry in Organic Synthesis; Open Access
Identification of a Dual Inhibitor of SRPK1 and CK2 that Attenuates Pathological Angiogenesis of Macular Degeneration in Mice
◆ 38
S. Morooka, M. Hoshina, I. Kii, T. Okabe, H. Kojima, N. Inoue, Y. Okuno, M. Denawa, S. Yoshida, J. Fukuhara, K. Ninomiya, T. Ikura, T. Furuya, T. Nagano, K. Noda, S. Ishida, T. Hosoya, N. Ito, N. Yoshimura, and *M. Hagiwara
Mol. Pharmacol. 2015, 88, 316.
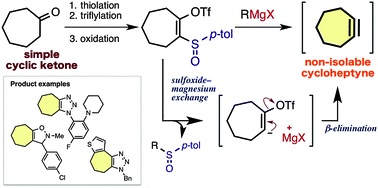
Generation of cycloheptynes and cyclooctynes via a sulfoxide–magnesium exchange reaction of readily synthesized 2-sulfinylcycloalkenyl triflates
◆ 37
S. Yoshida, F. Karaki, K. Uchida, *T. Hosoya
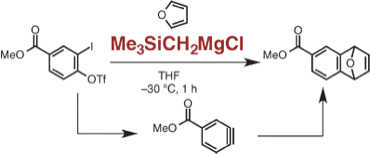
Generation of Arynes Using Trimethylsilylmethyl Grignard Reagent for Activation of ortho-Iodoaryl or ortho-Sulfinylaryl Triflates
◆ 36
S. Yoshida, K. Uchida, *T. Hosoya
Rectifier of aberrant mRNA splicing recovers tRNA modification in familial dysautonomia
◆ 35
M. Yoshida, N. Kataoka, K. Miyauchi, K. Ohe, K. Iida, S. Yoshida, T. Nojima, Y. Okuno, H. Onogi, T. Usui, A. Takeuchi, T. Hosoya, T. Suzuki, *M. Hagiwara
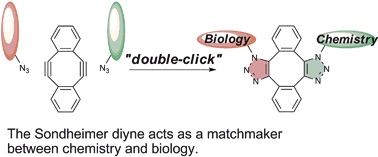
Substituted 5,6,11,12-Tetradehydrodibenzo[a,e]cyclooctenes: Syntheses, Properties, and DFT Studies of Substituted Sondheimer–Wong Diynes
◆ 34
F. Xu, L. Peng, K. Shinohara, T. Morita, S. Yoshida,
T. Hosoya, *A. Orita, J. Otera
J. Org. Chem. 2014, 79, 11592.
An Efficient generation method and remarkable reactivities of 3-triflyloxybenzyne
◆ 33
S. Yoshida, K. Uchida, K. Igawa, K. Tomooka, *T. Hosoya
Chem. Commun. 2014, 50, 15059.
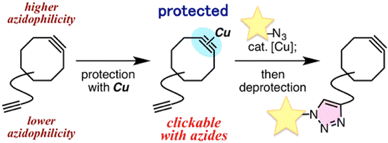
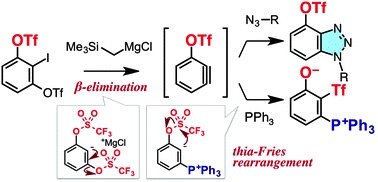
Transient Protection of Strained Alkynes from Click Reaction via Complexation with Copper
◆ 32
*S. Yoshida, Y. Hatakeyama, K. Johmoto, H. Uekusa,
*T. Hosoya
J. Am. Chem. Soc. 2014, 136, 13590.
https://doi.org/10.1021/ja507660x
Highlighted in Nature Chem., Organometallic News,
and Org. Chem. Highlights
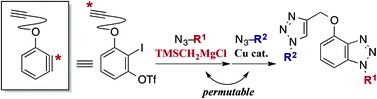
Modular synthesis of bis- and tris-1,2,3-triazoles by permutable sequential azide–aryne and azide–alkyne cycloadditions
◆ 31
S. Yoshida, T. Nonaka, T. Morita, *T. Hosoya
Org. Biomol. Chem. 2014, 12, 7489.
CDK9 inhibitor FIT-039 prevents replication of multiple DNA viruses
◆ 30
M. Yamamoto, H. Onogi, I. Kii, S. Yoshida, K. Iida,
H. Sakai, M. Abe, T. Tsubota, N. Ito, T. Hosoya,
*M. Hagiwara
J. Clin. Investig. 2014, 124, 3479.
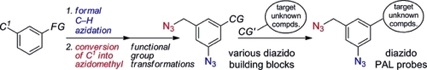
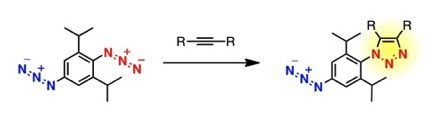
Formal C–H-Azidation-Based Shortcut to Diazido Building Blocks for the Versatile Preparation of Photoaffinity Labeling Probes
◆ 29
S. Yoshida, Y. Misawa, *T. Hosoya
Eur. J. Org. Chem. 2014, 3991
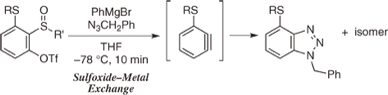
Generation of Arynes Triggered by the Sulfoxide–Metal Exchange Reaction of ortho-Sulfinylaryl Triflates
◆ 28
S. Yoshida, K. Uchida, *T. Hosoya
Luminescence enhancement of the catalytic 19 kDa protein (KAZ) of Oplophorus luciferase by three amino acid substitutions
◆ 27
*S. Inouye, J.-i. Sato, Y. Sahara–Miura, S. Yoshida,
H. Kurakata, *T. Hosoya
Biochemi. Biophys. Res. Commun. 2014, 445, 157.
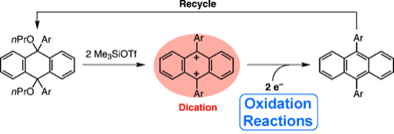
A New Organic Two-Electron Oxidant: 9,10-Diaryl-9,10-dihydroanthracene-9,10-bis(ylium)
◆ 26
*J. Ichikawa, H. Tanabe, S. Yoshida, T. Kawai, M. Shinjo, T. Fujita
Chem. Asian J. 2013, 8, 2588.
Development of bis-unsaturated ester aldehydes as amino-glue probes: Sequential double azaelectrocyclization as promising strategy for bioconjugation
◆ 25
*K. Tanaka, Y. Nakamoto, E. R. O. Siwu, A. R. Pradipta, K. Morimoto, T. Fujiwara, S. Yoshida, T. Hosoya, Y. Tamura, G. Hirai, M. Sodeoka, *K. Fukase
Org. Biomol. Chem. 2013, 11, 7326.
C6-Deoxy coelenterazine analogues as an efficient substrate for glow luminescence reaction of nanoKAZ: the mutated catalytic 19 kDa component of Oplophorus luciferase
◆ 24
*S. Inouye, J.-i. Sato, Y. Sahara–Miura, S. Yoshida,
H. Kurakata, *T. Hosoya
Biochem. Biophys. Res. Commun. 2013, 437, 23.
Synthesis of diverse aromatic oxophosphorus compounds by the Michaelis–Arbuzov-type reaction of arynes
◆ 23
S. Yoshida, *T. Hosoya
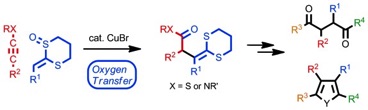
Copper-Catalyzed Extended Pummerer Reactions of Ketene Dithioacetal Monoxides with Alkynyl Sulfides and Ynamides with an Accompanying Oxygen Rearrangement
◆ 22
K. Murakami, J. Imoto, H. Matsubara, S. Yoshida,
*H. Yorimitsu, *K. Oshima
Chem. Eur. J. 2013, 23, 5625.
Expression, purification and luminescence properties of coelenterazine-utilizing luciferases from Renilla, Oplophorus and Gaussia: Comparison of substrate specificity for C2-modified coelenterazines
◆ 21
*S. Inouye, Y. Sahara–Miura, J.-i. Sato, R. Iimori,
S. Yoshida, *T. Hosoya
Protein Expr. Purif. 2013, 88, 150.
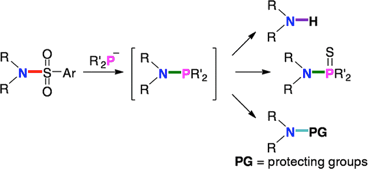
Nucleophilic Substitution Reaction at the Nitrogen of Arylsulfonamides with Phosphide Anion
◆ 20
S. Yoshida, K. Igawa, *K. Tomook
Palladium-Catalyzed Regio- and Stereoselective Hydrosilylation of Electron-Deficient Alkynes
◆ 19
*Y. Sumida, T. Kato, S. Yoshida, *T. Hosoya
Org. Lett. 2012, 14, 1552.
Remodeling of Actin Cytoskeleton in Mouse Periosteal Cells under Mechanical Loading Induces Periosteal Cell Proliferation during Bone Formation
◆ 18
D. Sakai, I. Kii, K. Nakagawa, H. N. Matsumoto, M. Takahashi, S. Yoshida, T. Hosoya, K. Takakuda, *A. Kudo
PLoS ONE 2011, 6, e24847.
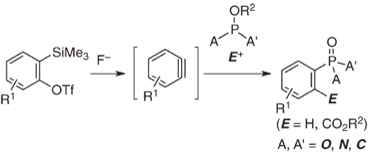
Enhanced clickability of doubly sterically-hindered aryl azides
◆ 17
S. Yoshida, A. Shiraishi, K. Kanno, T. Matsushita,
K. Johmoto, H. Uekusa, *T. Hosoya
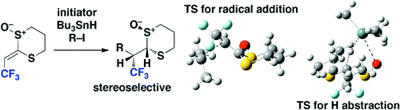
Intermolecular Reductive Radical Addition to 2-(2,2,2-Trifluoroethylidene)-1,3-dithiane 1-Oxide: Experimental and Theoretical Studies
◆ 16
R. Nakayama, H. Matsubara, D. Fujino, T. Kobatake,
S. Yoshida, *H. Yorimitsu, *K. Oshima
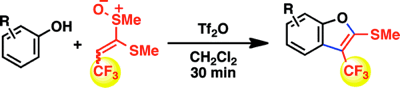
Synthesis of 3-Trifluoromethylbenzo[b]furans from Phenols via Direct Ortho Functionalization by Extended Pummerer Reaction
◆ 15
T. Kobatake, D. Fujino, S. Yoshida, *H. Yorimitsu,
*K. Oshima
J. Am. Chem. Soc. 2010, 132, 11838.
https://doi.org/10.1021/ja1030134
Highlighted in Synfacts and Org. Proc. Res. Dev.
Strain-promoted double-click reaction for chemical modification of azido-biomolecules
◆ 14
I. Kii, A. Shiraishi, T. Hiramatsu, T. Matsushita, H. Uekusa,
S. Yoshida, M. Yamamoto, A. Kudo, M. Hagiwara, *T. Hosoya
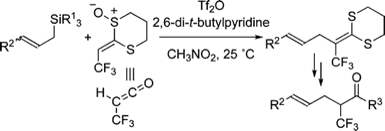
Reaction of 2-(2,2,2-Trifluoroethylidene)-1,3-dithiane 1-Oxide with Ketones under Pummerer Conditions and Its Application to the Synthesis of Trifluoromethyl-Substituted Five-Membered Heteroaromatics
◆ 13
T. Kobatake, S. Yoshida, *H. Yorimitsu, *K. Oshima
Angew. Chem., Int. Ed. 2010, 49, 2340.
https://doi.org/10.1002/anie.200906774
Highlighted in Org. Proc. Res. Dev.
Tin-Hydride-Mediated Radical Addition of Alkyl Halides to 2-Methylene-1,3-dithiane Monoxide as a Ketene Equivalent
◆ 12
S. Yoshida, *H. Yorimitsu, *K. Oshima
Heterocycles 2010, 80, 259.
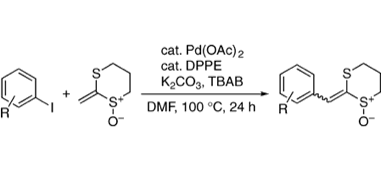
Palladium-catalyzed Mizoroki–Heck Reactions of 2-Methylene-1,3-dithiane 1-Oxide with Aryl Iodides
◆ 11
E. Morita, M. Iwasaki, S. Yoshida, *H. Yorimitsu,
*K. Oshima
Chem. Lett. 2009, 38, 624.
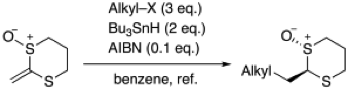
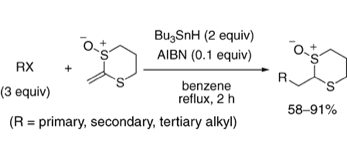
2-(2,2,2-Trifluoroethylidene)-1,3-dithiane Monoxide as a Trifluoromethylketene Equivalent
◆ 10
S. Yoshida, *H. Yorimitsu, *K. Oshima
Radical Addition of Alkyl Halides to 2-Methylene-1,3-dithiane Monoxide as a Ketene Equivalent
◆ 9
S. Yoshida, *H. Yorimitsu, *K. Oshima
Chem. Lett. 2009, 8, 248.
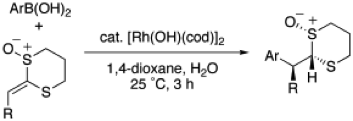
2-Alkylidene-1,3-dithiane Monoxides as Activated Alkenes in Rhodium-Catalyzed Addition Reaction of Arylboronic Acids
◆ 8
S. Yoshida, *H. Yorimitsu, *K. Oshima
Heterocycles 2008, 76, 679.
Synthesis of Bulky Arylphosphanes by Rhodium-Catalyzed Formal [2+2+2] Cycloaddition Reaction and Their Use as Ligands
◆ 7
T. Kobatake, A. Kondoh, S. Yoshida, *H. Yorimitsu,
*K. Oshima
Chem. Asian J. 2008, 3, 1613.
Extended Pummerer Reaction of Arylketene Dithioacetal Monoxides with Aromatic Compounds by Means of Trifluoromethanesulfonic Anhydride
◆ 6
S. Yoshida, *H. Yorimitsu, *K. Oshima
Chem. Lett. 2008, 37, 786.
Synthesis of Benzo[b]thiophenes by Cyclization of Arylketene Dithioacetal Monoxides under Pummerer-like Conditions
◆ 5
S. Yoshida, *H. Yorimitsu, *K. Oshima
Zirconocene-Catalyzed Alkylative Dimerization of 2-Methylene-1,3-dithiane via a Single Electron Transfer Process to Provide Symmetrical vic-Bis(dithiane)s
◆ 4
S. Yoshida, *H. Yorimitsu, *K. Oshima
J. Organomet. Chem. 2007, 692, 3110.

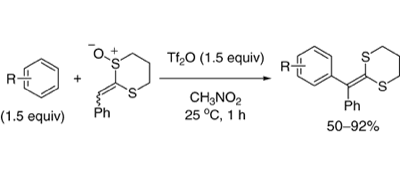
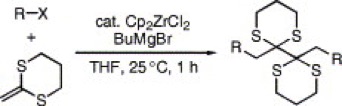
Rhodium-Catalyzed Addition of Arylboronic Acids to 2-Methylene-1,3-dithiane Monoxide
◆ 3
S. Yoshida, *H. Yorimitsu, *K. Oshima
Synlett 2007, 1622.
https://doi.org/10.1055/s-2007-980373
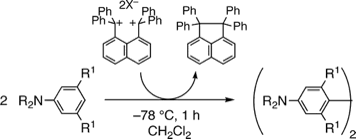
Naphthalene-1,8-diylbis(diphenylmethylium) as an Organic Two-Electron Oxidant: Benzidine Synthesis via Oxidative Self-Coupling of N,N-Dialkylanilines
◆ 2
T. Saitoh, S. Yoshida, *J. Ichikawa
1,8-Bis(diphenylmethylium)naphthalenediyl Dication as an Organic Oxidant: Synthesis of Benzidines via Self-Coupling of N,N-Dialkylanilines
◆ 1
T. Saitoh, S. Yoshida, *J. Ichikawa
Orga. Lett. 2004, 6, 4563.
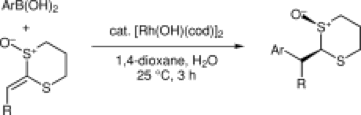
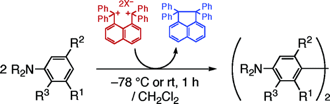
Publication
Original Paper (2020~present / 2016~2019 / before 2015)/Review, book, etc
Faculty of Advanced Engineering
Department of Biological Science and Technology




