Cell-based HTS identifies a chemical chaperone for preventing ER protein aggregation and proteotoxicity
◆ 84
K. Kitakaze, S. Taniuchi, E. Kawano, Y. Hamada, M. Miyake, M. Oyadomari, H. Kojima, H. Kosako, T. Kuribara, S. Yoshida, T. Hosoya, *S. Oyadomari
eLife 2019, 8, e43302.
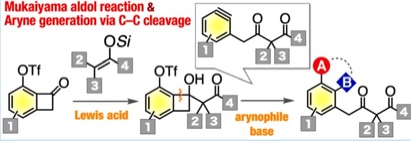
Synthesis of Diverse γ-Aryl-β-ketoesters via Aryne Intermediates Generated by C–C Bond Cleavage
◆ 83
K. Uchida, Y. Minami, *S. Yoshida, *T. Hosoya
Org. Lett. 2019, 21, 9019.
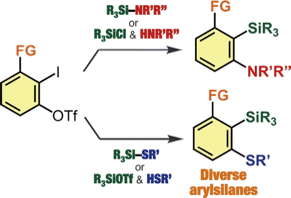
Facile Synthesis of Diverse 2,6-Disubstituted Arylsilanes via Silylamination and Silylsulfanylation of Aryne Intermediates Generated from o-Iodoaryl Triflates
◆ 82
Y. Nakamura, S. Ozawa, *S. Yoshida, *T. Hosoya
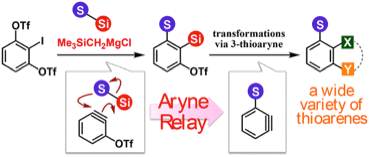
3-Thioaryne Intermediates for the Synthesis of Diverse Thioarenes
◆ 81
Y. Nakamura, Y. Miyata, K. Uchida, *S. Yoshida,
*T. Hosoya
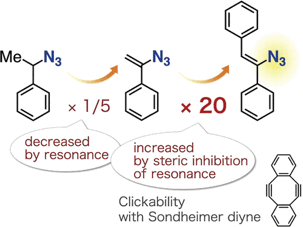
Effect of Resonance on the Clickability of Alkenyl Azides in the Strain-promoted Cycloaddition with Dibenzo-fused Cyclooctynes
◆ 80
*S. Yoshida, S. Goto, Y. Nishiyama,Y. Hazama, M. Kondo, T. Matsushita, *T. Hosoya
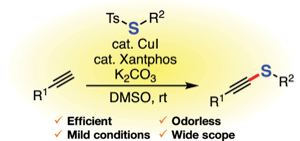
Synthesis of Alkynyl Sulfides by Copper-Catalyzed Thiolation of Terminal Alkynes Using Thiosulfonates
◆ 79
K. Kanemoto, *S. Yoshida, and *T. Hosoya
Org. Lett. 2019, 21, 3172.
https://doi.org/10.1021/acs.orglett.9b00875
Most read article of OL in April 2019.
Facile Synthesis of Diverse o-Iodoaryl Triflates from o-Silylaryl Triflates by Aluminum-Mediated Desilyliodination
◆ 78
*S. Yoshida, Y. Hazama, K. Kanemoto, Y. Nakamura,
*T. Hosoya
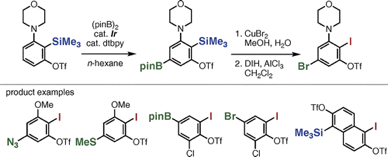
Modular Synthesis of Unsymmetrical Doubly-ring-fused Benzene Derivatives Based on a Sequential Ring Construction Strategy Using Oxadiazinones as a Platform Molecule
◆ 77
T. Meguro, S. Chen, K. Kanemoto, *S. Yoshida, *T. Hosoya
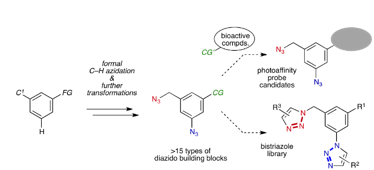
Synthesis of Diverse 3-Azido-5-(azidomethyl)benzene Derivatives via Formal C–H Azidation and Functional Group-Selective Transformations
◆ 76
Y. Nishiyama, Y. Misawa, Y. Hazama, K. Oya, *S. Yoshida, *T. Hosoya
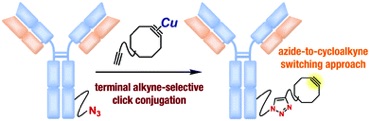
A facile preparation of functional cycloalkynes by an azide-to-cycloalkyne switching approach
◆ 75
*S. Yoshida, T. Kuribara, H. Ito, T. Meguro, Y. Nishiyama,
F. Karaki, Y. Hatakeyama, Y. Koike, I. Kii, *T. Hosoya
Ligand binding to human prostaglandin E receptor EP4 at the lipid-bilayer interface
◆ 74
Y. Toyoda, K. Morimoto, R. Suno, S. Horita, K. Yamashita, K. Hirata, Y. Sekiguchi, S. Yasuda, M. Shiroishi, T. Shimizu, Y. Urushibata, Y. Kajiwara, T. Inazumi, Y. Hotta, H. Asada, T. Nakane, Y. Shiimura, T. Nakagita, K. Tsuge, S. Yoshida, T. Kuribara, T. Hosoya, Y. Sugimoto, N. Nomura, M. Sato, T. Hirokawa, K. Masahiro, T. Murata, K. Takayama, M. Yamamoto, *S. Narumiya, *S. Iwata, and *T. Kobayashi
Nature Chem. Biol. 2019, 15, 18.
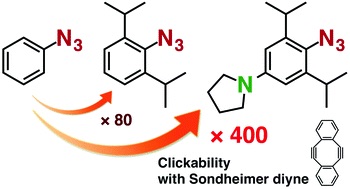
Further enhancement of the clickability of doubly sterically-hindered aryl azides by para-amino substitution
◆ 73
S. Yoshida, J. Tanaka, Y. Nishiyama, Y. Hazama, T. Matsushita, *T. Hosoya
Chem. Commun. 2018, 54, 13499.
https://doi.org/10.1039/C8CC05791E
Open Access; Selected as inside back cover.
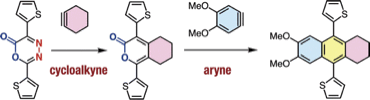
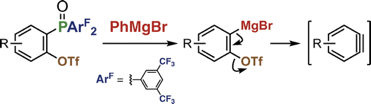
Generation of Arynes by Selective Cleavage of a Carbon–Phosphorus Bond of o-(Diarylphosphinyl)aryl Triflates Using a Grignard Reagent
◆ 72
Y. Nishiyama, S. Kamada, *S. Yoshida, *T. Hosoya
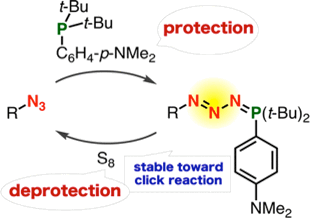
Transient Protection of Organic Azides from Click Reactions with Alkynes by Phosphazide Formation
◆ 71
T. Meguro, *S. Yoshida, K. Igawa, K. Tomooka, *T. Hosoya
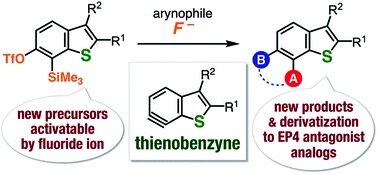
Expanding the synthesizable multisubstituted benzo[b]thiophenes via 6,7-thienobenzynes generated from o-silylaryl triflate-type precursors
◆ 70
*S. Yoshida, T. Kuribara, T. Morita, T. Matsuzawa,
K. Morimoto, T. Kobayashi, *T. Hosoya
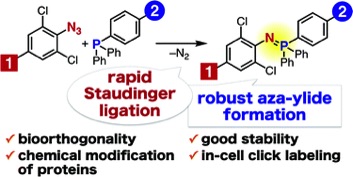
Staudinger reaction using 2,6-dichlorophenyl azide derivatives for robust aza-ylide formation applicable to bioconjugation in living cells
◆ 69
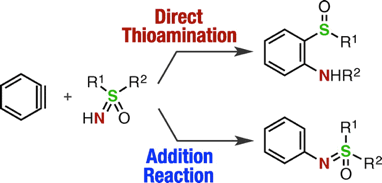
T. Meguro, N. Terashima, H. Ito, Y. Koike, I. Kii,
*S. Yoshida, and *T. Hosoya
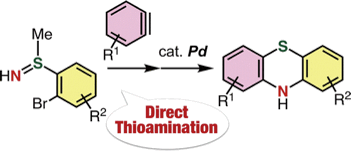
Synthesis of Diverse Phenothiazines by Direct Thioamination of Arynes with S-(o-Bromoaryl)-S-methylsulfilimines and Subsequent Intramolecular Buchwald–Hartwig Amination
◆ 68
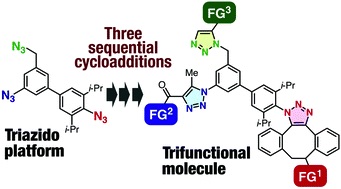
T. Matsuzawa, K. Uchida, *S. Yoshida, *T. Hosoya
Convergent synthesis of trifunctional molecules by three sequential azido-type-selective cycloadditions
◆ 67
S. Yoshida, K. Kanno, I. Kii, Y. Misawa, M. Hagiwara,
*T. Hosoya
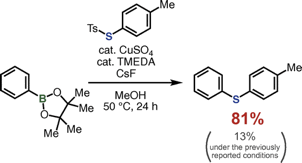
Modified Conditions for Copper-Catalyzed ipso-Thiolation of Arylboronic Acid Esters with Thiosulfonates
◆ 66
K. Kanemoto, *S. Yoshida, *T. Hosoya
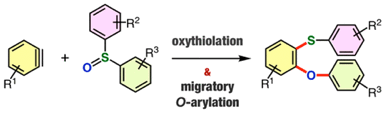
Synthesis of Diverse o-Arylthio-Substituted Diaryl Ethers by Direct Oxythiolation of Arynes with Diaryl Sulfoxides Involving Migratory O-Arylation
◆ 65
T. Matsuzawa, K. Uchida, *S. Yoshida, *T. Hosoya
Construction of Condensed Polycyclic Aromatic Frameworks through Intramolecular Cycloaddition Reactions Involving Arynes Bearing an Internal Alkyne Moiety
◆ 64
*S. Yoshida, K. Shimizu, K. Uchida, Y. Hazama, K. Igawa, K. Tomooka, *T. Hosoya
Comparison of Pharmacokinetics of Newly Discovered Aromatase Inhibitors by A Cassette Microdosing Approach in Healthy Japanese Subjects
◆ 63
H. Kusuhara, T. Takashima, H. Fujii, T. Takashima,
M. Tanaka, A. Ishii, S. Tazawa, K. Takahashi, K. Takahashi,
H. Tokai, T. Yano, M. Kataoka, A. Inano, S. Yoshida,
T. Hosoya, Y. Sugiyama, S. Yamashita, T. Hojo,
*Y. Watanabe
Drug Metab. Pharmacokinet. 2017, 32, 293.
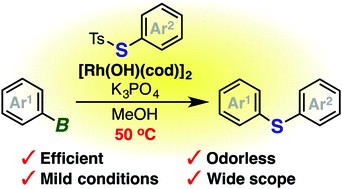
Rhodium-catalyzed odorless synthesis of diaryl sulfides from borylarenes and S-aryl thiosulfonates
◆ 62
K. Kanemoto, Y. Sugimura, S. Shimizu, *S. Yoshida,
*T. Hosoya
Prenatal neurogenesis induction therapy normalizes brain structure and function in Down syndrome mice
◆ 61
A. Nakano-Kobayashi, T. Awaya, I. Kii, Y. Sumida,
Y. Okuno, S. Yoshida, T. Sumida, H. Inoue, T. Hosoya,
*M. Hagiwara
Proc. Natl. Acad. Sci. USA 2017, 114, 10268.
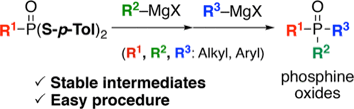
Synthesis of Unsymmetrical Tertiary Phosphine Oxides via Sequential Substitution Reaction of Phosphonic Acid Dithioesters with Grignard Reagents
◆ 60
Y. Nishiyama, Y. Hazama, *S. Yoshida, *T. Hosoya
Development of an orally available inhibitor of CLK1 for skipping a mutated dystrophin exon in Duchenne muscular dystrophy
◆ 59
Y. Sako, K. Ninomiya, Y. Okuno, M. Toyomoto, A. Nishida, Y. Koike, K. Ohe, I. Kii, S. Yoshida, N. Hashimoto,
T. Hosoya, M. Matsuo, *M. Hagiwara
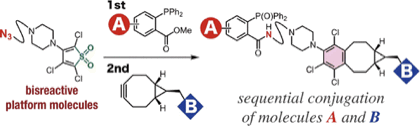
Sequential Molecular Conjugation using Thiophene S,S-Dioxides Bearing a Clickable Functional Group
◆ 58
T. Meguro, *S. Yoshida, *T. Hosoya
Chem. Lett. 2017, 46, 1137.
https://doi.org/10.1246/cl.170426
Selected as Editor’s choice; Open Access
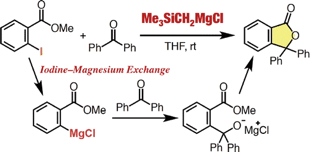
Facile Synthesis of Phthalides from Methyl ortho-Iodobenzoates and Ketones via an Iodine–Magnesium Exchange Reaction Using a Silylmethyl Grignard Reagent
◆ 57
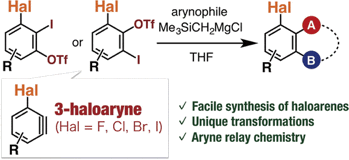
Y. Nakamura, *S. Yoshida, *T. Hosoya
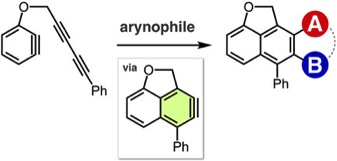
Enhancing the Synthetic Utility of 3-Haloaryne Intermediates by their Efficient Generation from Readily Synthesizable ortho-Iodoaryl Triflate-type Precursors
◆ 56
*S. Yoshida, A. Nagai, K. Uchida, *T. Hosoya
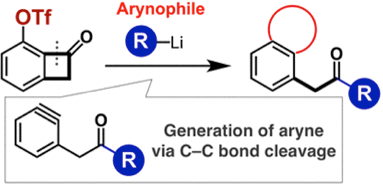
Three-Component Coupling of Triflyloxy-Substituted Benzocyclobutenones, Organolithium Reagents, and Arynophiles Promoted by Generation of Aryne via Carbon–Carbon Bond Cleavage
◆ 55
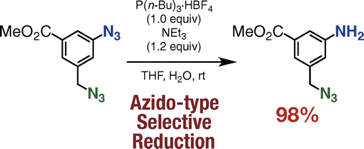
K. Uchida, *S. Yoshida, *T. Hosoya
Aromatic Azido-selective Reduction via the Staudinger Reaction Using Tri-n-butylphosphonium Tetrafluoroborate with Triethylamine
◆ 54
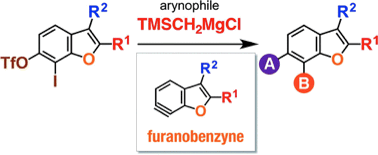
T. Meguro, *S. Yoshida, *T. Hosoya
Facile Synthesis of Multisubstituted Benzo[b]furans via 2,3-Disubstituted 6,7-Furanobenzynes Generated from ortho-Iodoaryl Triflate-type Precursors
◆ 53
T. Morita, Y. Nishiyama, *S. Yoshida, *T. Hosoya
Facile Diversification of Simple Benzo[b]thiophenes via Thienobenzyne Intermediates
◆ 52
T. Morita, *S. Yoshida, M. Kondo, T. Matsushita,
*T. Hosoya
Reactions of Arynes with Sulfoximines: Formal Sulfinylamination vs N-Arylation
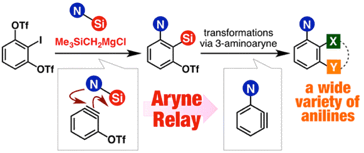
◆ 51
*S. Yoshida, H. Nakajima, K. Uchida, T. Yano, M. Kondo,
T. Matsushita, *T. Hosoya
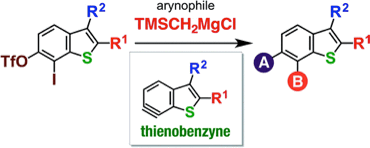
Aryne Relay Chemistry en Route to Aminoarenes: Synthesis of 3-Aminoaryne Precursors via Regioselective Silylamination of 3-(Triflyloxy)arynes
◆ 50
*S. Yoshida, Y. Nakamura, K. Uchida, Y. Hazama,
*T. Hosoya
The mevalonate pathway regulates primitive streak formation via protein farnesylation
◆ 49
Y. Okamoto-Uchida, R. Yu, N. Miyamura, N. Arima, M. Ishigami-Yuasa, H. Kagechika, S. Yoshida, T. Hosoya, M. Nawa, T. Kasama, Y. Asaoka, R. W. Alois, U. Elling, J. M. Penninger, S. Nishina, N. Azuma, *H. Nishina
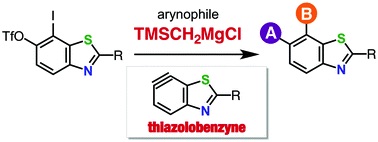
Thiazolobenzyne: a versatile intermediate for multisubstituted benzothiazoles
◆ 48
S. Yoshida, T. Yano, Y. Nishiyama, Y. Misawa, M. Kondo,
T. Matsushita, K. Igawa, K. Tomooka, *T. Hosoya
Controlled Generation of 3-Triflyloxyarynes
◆ 47
K. Uchida, *S. Yoshida, *T. Hosoya
Synthesis 2016, 48, 4099.
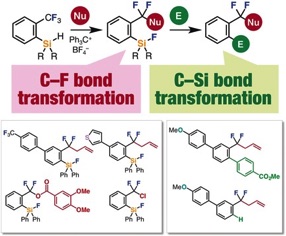
Single C–F Bond Cleavage of Trifluoromethylarenes with an ortho-Silyl Group
◆ 46
*S. Yoshida, K. Shimomori, Y. Kim, *T. Hosoya
Angew. Chem., Int. Ed. 2016, 55, 10406.
https://doi.org/10.1002/anie.201604776
Selected as hot paper and inside cover
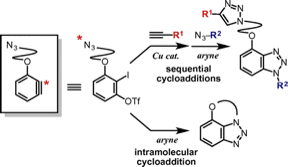
Synthesis of Diverse Benzotriazoles from Aryne Precursors Bearing an Azido Group via Inter- and Intramolecular Cycloadditions
◆ 45
S. Yoshida, T. Morita, *T. Hosoya
Selective inhibition of the kinase DYRK1A by targeting its folding process
◆ 44
*I. Kii, Y. Sumida, T. Goto, R. Sonamoto, Y. Okuno, S. Yoshida, T. Kato-Sumida, Y. Koike, M. Abe, Y. Nonaka, T. Ikura, N. Ito, H. Shibuya, T. Hosoya, *M. Hagiwara
Publication
Original Paper (2020~present / 2016~2019 / before 2015)/Review, book, etc
Faculty of Advanced Engineering
Department of Biological Science and Technology




